Sf6 Polar Or Nonpolar
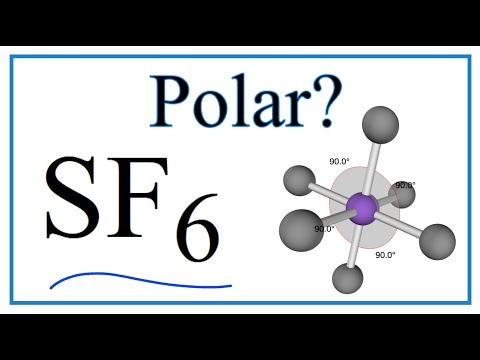
Why is SF6 non-polar?
There are two polar requirements.
1: Must have polar connection. The SF connection is polar.
2: There should be a lack of balance which allows you to reduce the molecule a little and a little + half. The molecule is octahedral, meaning fully compatible.
If you are familiar with vectors, the vector amount of bond polarity is added to z.
NF6 polar or non-polar.
Sf6 Polar Or Nonpolar
source https://howtodiscuss.com/t/sf6-polar-or-nonpolar/74145/1

No comments:
Post a Comment